Background: Reduced or low-dose direct oral anticoagulants (DOACs) have been studied in randomized control trials for the extended prevention of venous thromboembolism (VTE) after 6 months of treatment at full, therapeutic doses. The real-world effectiveness as well as utilization of this approach in clinical practice compared to continuing at therapeutic doses is unclear.
Aims: Examine the effectiveness, safety and utilization of reduced-dose DOACs in cancer patients and overweight patients.
Methods: Consecutive patients with VTE were identified using the Mayo Clinic VTE Registry from March 1st, 2013 to December 31st, 2021. Patients were followed prospectively for outcomes of VTE recurrence, death, major bleeding and clinically relevant non major bleeding (CRNMB) either in person or by survey. Patients with recurrent VTE or bleeding during the first 3 months were excluded from further analysis. After completion of anticoagulation treatment for 3 months with either rivaroxaban or apixaban, patients continuing anticoagulation were evaluated in a nested case-control study.
Results: A total of 404 patients (283 (70%) on apixaban and 121 (30%) on rivaroxaban) were identified in the low dose DOAC and 3060 in the full dose anticoagulation group. Overall, the mean age was 60.7 years (SD 14.4), mean weight was 89.9 kg (SD 23.8) and 55.1% were males. Within the full cohort, patients with active cancer were less likely to be prescribed low dose DOACs (HR 0.56; 95%CI 0.42-0.73; p<0.001). However, low dose DOAC prescription was more likely in patients with pulmonary embolism alone (HR 1.48; 95%CI 1.21-1.80; p<0.001) or in combination with DVT (HR 1.54; 95%CI 1.25,1.90; p<0.001). Patients transitioned to low dose DOACs had similar age, sex and weight compared to the full dose anticoagulation group, however, fewer patients with weight ≥120 kg had reduced dosing (HR 0.68; 95%CI 0.46,0.99; p=0.046).
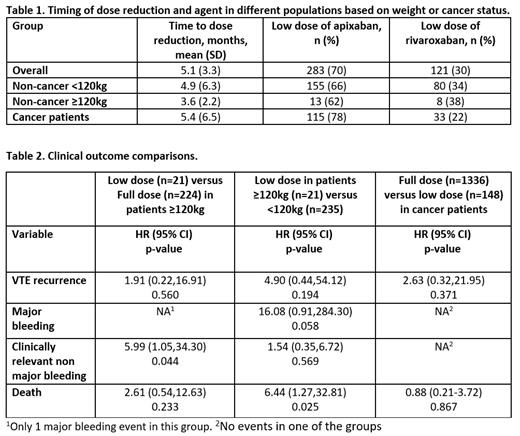
The mean time to start of a low dose DOAC was 5.1 months overall, 5.4 months in cancer patients, and 3.6-4.9 months in non-cancer patients (Table 1). Non-cancer patients had significantly lower transition numbers in those ≥120kg (HR 0.62; 95%CI 0.40,0.97; p=0.038).
In non-cancer patients In comparing weight, ≥120kg to <120kg, in the low dose group, only death was statistically different with higher risk in those ≥120kg (HR 6.44; 95%CI1.27,32.81; p=0.025).
Cancer patients transitioned to low dose DOACs around 5 months on average with a median of 3.3 months. Apixaban continued to be the dominant DOAC used (78%) in the low dose group. The cancers with the most patients on low dose DOACs include lymphoma, prostate, melanoma, ovarian, and breast. There was no significant difference in VTE recurrence or death in the cancer group on different DOAC doses.
Conclusion: Our results show that anticoagulant prescribers are not always waiting until 6 months to transition to low doses and low doses are being utilized in cancer patients despite lack of cancer-specific studies during the analyzed time period. Although the sample size limits the precision of this analysis, we did not find statistically significant increases in VTE recurrence with low dose DOACs in either cancer patients or with elevated body weight. We did find CRNMB but not MB was observed more frequently in weights above 120kg, possibly from unadjusted confounding in this unadjusted analysis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal